(Carrick Pharmacy News) The US Food and Drug Administration (FDA) has approved a new plaque psoriasis drug.
This new medication is found under the brand name Sotyktu. Its active ingredient is deucravacitinib.
This drug is approved to treat adults with moderate to severe plaque psoriasis who will likely benefit from injection, pills or ultraviolet treatment.
It hasn’t been tested in those younger than 18 years of age.
Estimates suggest that more than 8 million people in the US have psoriasis.
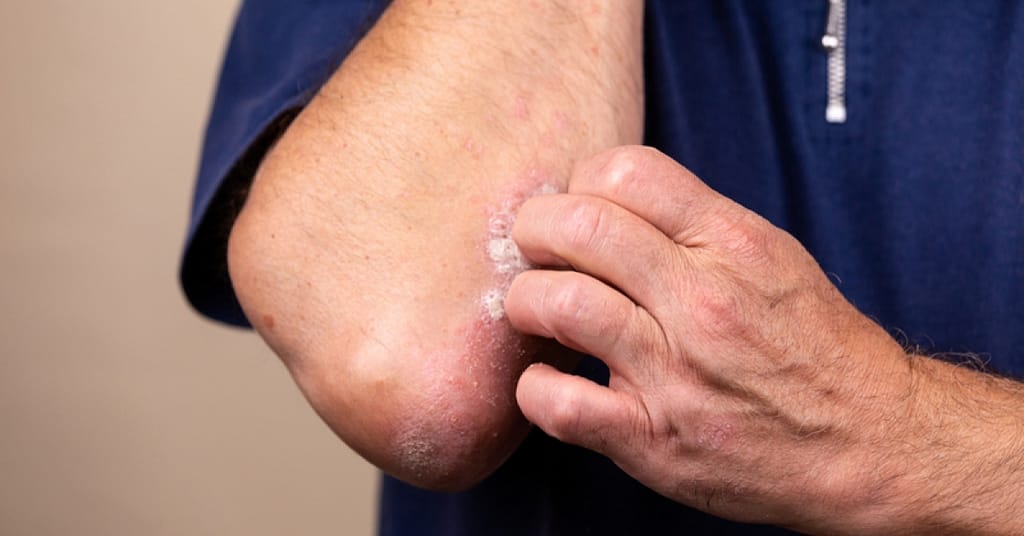
And plaque psoriasis is considered the most common type of psoriasis. In fact, an estimated 80 percent of those with psoriasis have plaque psoriasis.
Plaque psoriasis is characterized by dry, itchy, raised skin patches (plaques).
According to the manufacturer of Sotyktu, this drug works to treat plaque psoriasis by targeting a molecule known as TYK2. This target is critically important because it is where psoriasis starts.
It is available in pill form and is to be taken once a day, with or without food. Do not crush, cut or chew tablets.
Common side effects of Sotyktu included the common cold, sore throat, sinus infection, sores on the lips or in the mouth and acne.
This medication can increase your risk for infections or make an infection worse. That’s why it is important to tell your doctor if you:
- Are currently being treated for an infection
- Have had an infection that doesn’t seem to go away
- Have tuberculosis or have been in close contact with someone with TB
- Have or have had hepatitis B or C
- Think you have an infection
Serious side effects include the potential for changes in certain laboratory test results, such as liver enzymes.
Recipients of this drug should not get live vaccines while they are taking it.
This approval was granted to Bristol Myers Squibb. Speak with your healthcare provider if you have any questions.