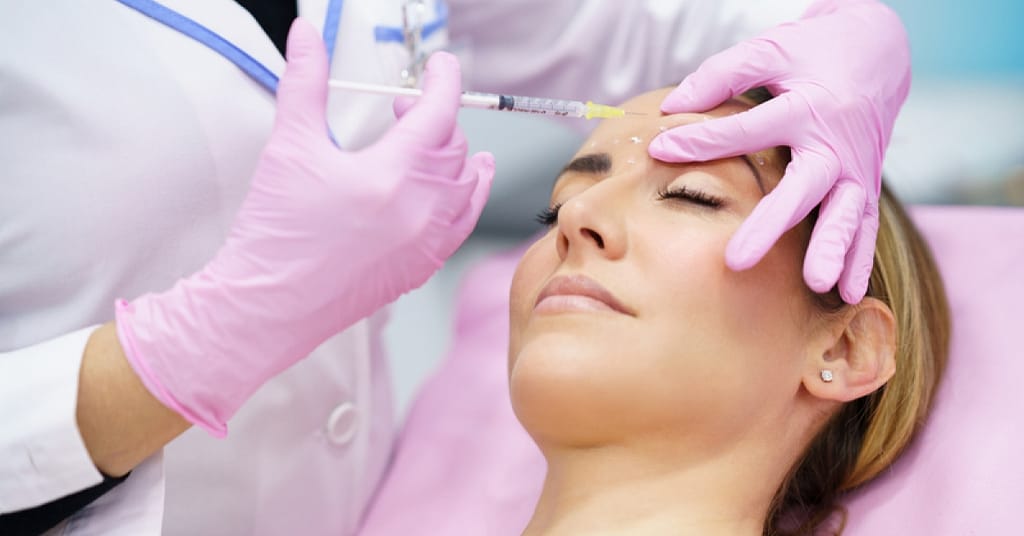
(Carrick Pharmacy News) The US Food and Drug Administration (FDA) has approved a new anti-wrinkle injection.
This new drug is found under the name Daxxify. Its active ingredient is daxibotulinumtoxinA.
It is currently approved to temporarily improve the look of moderate to severe frown lines between the eyebrows in adults.
In terms of frequency, this drug is not to be injected more than one time every three months.
This approval is highly awaited. And with good reason — according to the manufacturer, Daxxify’s effects have been shown to last longer than those of Botox.
The company claims that, according to the studies of the drug, Daxxify can sustain for six months when compared to the three months seen with Botox injections.
During clinical studies, some patients saw results at nine months.
The first drug of its kind, Daxxify uses peptide exchange technology. The toxin, that works to paralyzes the muscle, is attached to a protein. This protein helps to extend the effect that this drug has on the muscles. As a result, people will need it less frequently.
In other words, people can get as few as two treatments per year.
The most common side effects include eyelid drooping, headache and loss of the ability to move the muscles in your face.
This medication can cause loss of strength or result in muscle weakness, blurry vision or even drooping eyelids. This can happen within hours to weeks after this drug is injected. If this happens, do not drive a car, operate machinery or perform other dangerous activities.
Not everyone can get Daxxify. Speak with your healthcare provider if this is an option for you. Furthermore, tell your healthcare provider about all of the medications you are taking. This includes prescriptions, over-the-counter drugs, herbal treatments and supplements.
This medication’s approval was granted to Revance Therapeutics, Inc.