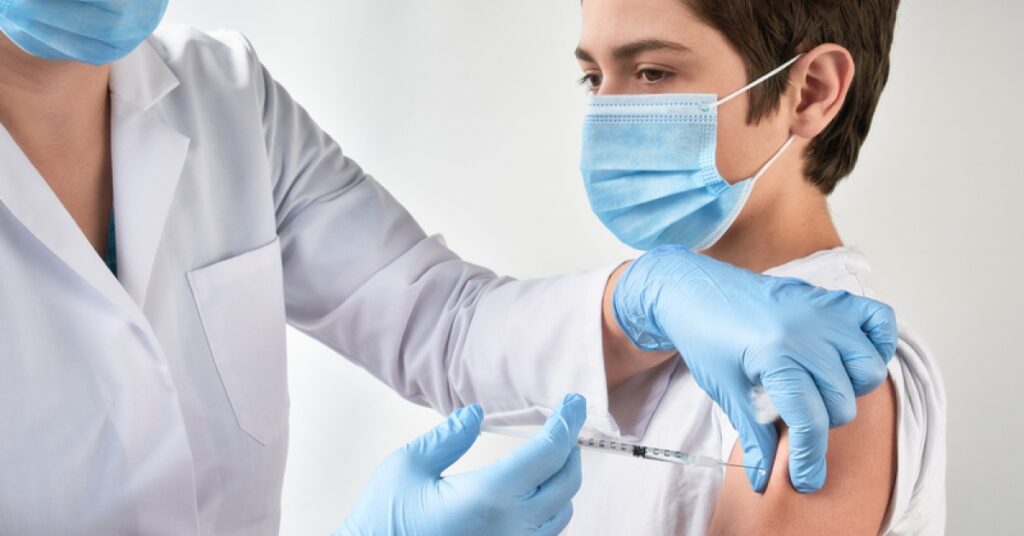
(Carrick Pharmacy News) The US Food and Drug Administration (FDA) extends authorization for the COVID-19 vaccine, Novavax.
This authorizations expands the COVID-19 vaccines available to kids.
Earlier in the summer, the FDA authorized Novavax COVID-19 Vaccine, Adjuvanted for those 18 years of age and older. This vaccine was not authorized for kids under the age of 18 — until now.
As of August 19, 2022, Novavax COVID-19 Vaccine, Adjuvanted is now authorized for individuals 12 through 17 years of age.
On August 22nd, the Centers for Disease Control and Prevention (CDC) released a recommendation. The CDC Director Rochelle P. Walensky, M.D., M.P.H., signed a decision memo for Novavax’s COVID-19 vaccine to be used as another primary series option for adolescents ages 12 through 17.
This vaccine is given as a two-dose primary series, three weeks apart. This vaccine works differently than that of the Pfizer and Moderna vaccines.
COVID-19 Vaccine, Adjuvanted is not a (mRNA) vaccine. Instead this vaccine packages harmless proteins of the COVID-19 virus plus Matrix-M adjuvant which helps the immune system respond to the virus in the future.
The CDC notes this type of vaccine, which uses proteins, have been used in the US for more than 30 years.
The most common side effects of this vaccine seen in adults included injection site reactions (pain/tenderness, redness and swelling), feeling tired, muscle/joint pain, headache, nausea/vomiting and fever.
The FDA and the CDC have processes in place to continually monitor this vaccine’s safety.
Furthermore, Novavax Inc. is to continue their clinical trials to gather more safety and effectiveness data.
If you have questions about any of the available COVID-19 vaccines, reach out to your doctor or community pharmacist.