(Carrick Pharmacy News) The US Food and Drug Administration (FDA) issues warning letters to three well known companies.
These warning letters have been sent to companies who are selling mole and skin tag removal products that have not been approved.
None of these products have been evaluated by the FDA for safety, effectiveness or quality.
As a result, these companies are in violation of Federal Food, Drug, and Cosmetic Act (FD&C Act).
To date, there are no over-the-counter products that are FDA approved to remove moles and skin tags.
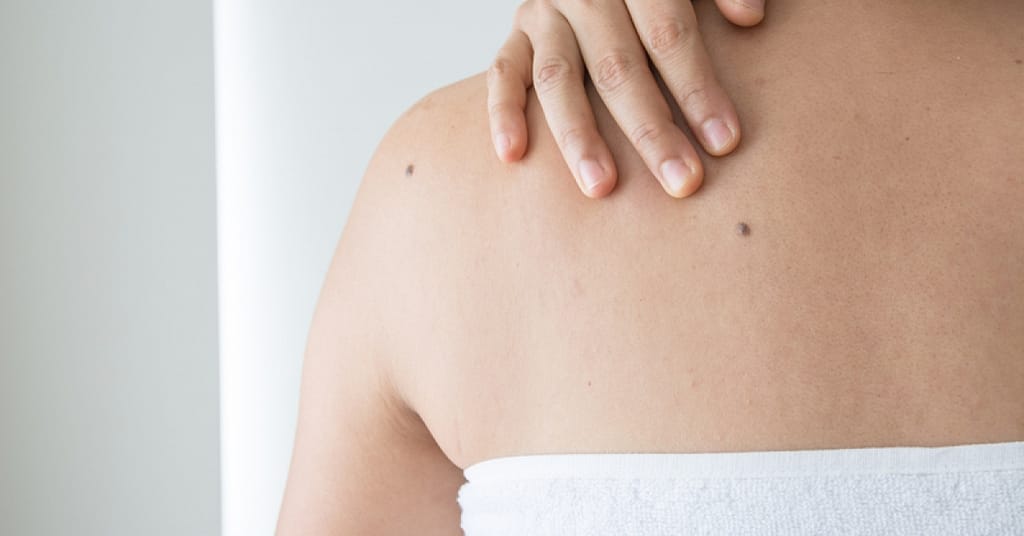
As we age, we may notice some new growths. You may notice new moles, seborrheic keratoses (wart-like growths) and skin tags.
It is recommended you get your skin evaluated, especially moles, by a health care provider.
According to the FDA, if you remove skin growths, like moles, or change the way they look, it becomes harder for health care providers to check for skin cancer. In other words, removing moles at home can lead to delayed cancer detection and in turn, treatment.
Furthermore, if skin cancer is not completely removed, the cancer can continue to grow and even spread.
Also, trying to remove moles and skin tags at home can result in skin injury, infection requiring antibiotics and scarring.
The FDA states these products often don’t remove the lesion or just removes part of it. And if the lesion is removed, you may see discoloration of the skin or even scarring.
The three companies who received a warning letter are:
- Amazon.com
- Ariella Naturals
- Justified Laboratories
These companies are selling a variety of products that include gels, liquids, ointments and sticks. These products may contain high amounts of salicylic acid or other harmful ingredients.
The agency also warns consumers about products claiming to be “natural,” “herbal” or “organic.” These labels do not mean they are harmless.
These three companies have been given 15 days to respond to the FDA with the actions they have taken to address any violations.
Consumers are recommended to report any adverse events to the agency’s MedWatch Adverse Event Reporting program.
It is recommended you check your skin regularly for changes in moles or for a new colored area. When you check your skin often, you will familiarize yourself with what’s normal.
If you have any concerns, speak with a dermatologist.