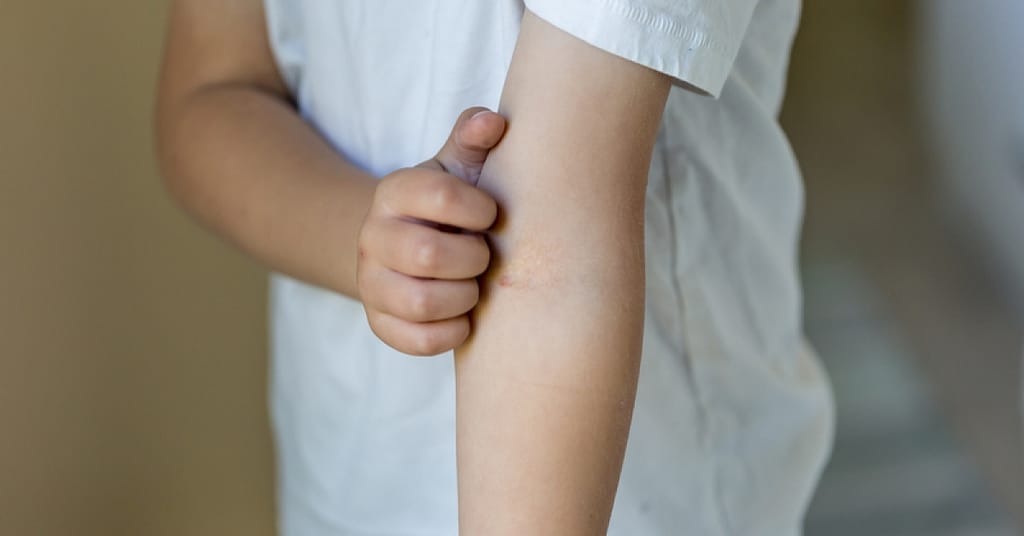
(Carrick Pharmacy News) The US Food and Drug Administration (FDA) has approved a new eczema treatment for kids.
This is the first biologic medicine for kids (aged 6 months to 5 years) with moderate to severe atopic dermatitis (eczema).
This new approval is found under the name Dupixent (dupilumab).
It is available as an injection and is to be administered under the skin every four weeks.
The dose is based on weight.
Dupilumab is already an approved treatment for eczema for kids 6 years and older. It wasn’t an option for kids younger than 6 years old until this most recent approval.
This medication is specifically for those whose eczema is not well-controlled with topical medications. It is also approved for those who can’t be treated with topical medications.
Dupilumab can be used with or without topical steroids.
Common side effects included reactions at the injection site, eye and eyelid inflammation and cold sores in the mouth or lips.
Speak with your health care provider if you have any questions.